What Controls The Direction Of A Molecule, Such As Oxygen, Involved In Passive Transport?
The cardiovascular or circulatory system is designed to ensure the survival of all cells of the body at every moment and it does this past maintaining the immediate chemical environment of each prison cell in the body (i.e., the interstitial fluid) at a limerick appropriate for that cell's normal function. The term "homeostasis" is used to denote the approximate constancy of the internal environment (Claude Bernard, 1866).
Showtime consider the simple hypothetical case of a unmarried spherical cell suspended in a large (>times the jail cell book), well-stirred volume of aqueous medium in equilibrium with room air and containing other nutrients. Oxygen availability is ofttimes a limiting gene for cell survival, and it is by and large supplied to a cell by passive diffusion. As oxygen molecules diffuse into the cell, they are consumed, so that there is a progressive autumn in oxygen concentration from the surface of the cell to the everyman concentration which occurs at the centre of the cell. For a spherical jail cell with a typicaldiffusion coefficient for oxygen (≈10−5 cmtwo/south) and an oxygen consumption of resting skeletal muscle (≈10−two ml O2 cm−3 min−1), the disquisitional size (radius) which is only adequately supplied with oxygen from the surrounding medium is virtually one mm. Thus, we find that diffusion puts an upper limit on the size of cells in regard to their need for oxygen.
Although diffusion is an efficient transport process over short distances (<100 μm) equally seen by the average time required for a molecule to diffuse a distance 10 (t ≈ ten 2/2d), how tin can a much larger multicellular organism, such as the human torso containing most 100 × 1012 cells, be fairly supplied with oxygen? For mammals, the bathing medium for cells is water and total body water is about lx% of body weight. For a 70-kg person, full body water is distributed among three compartments with the following approximate volumes: intracellular ≈23 50 (33% of trunk weight); interstitial ≈xvi l (22.v% of body weight); and circulating plasma ≈three l (iv.5% of torso weight). Cells are bathed in interstitial fluid (ISF), simply interstitial fluid book is only a little more than half the intracellular fluid volume. Thus, ISF cannot be considered a large reservoir of fluid, and its composition is directly influenced past cellular metabolism.
An organism is faced with the following problem: How can the limerick of ISF be maintained near its desired value? The solution of this problem is to introduce a circulatory organisation which continuously refreshes the ISF by putting it in intimate contact with "fresh, reconditioned" fluid (i.east., arterial claret). The circulating claret must be brought close to the cells (&#threescore;10 μm) since food and metabolic waste product exchange takes place by passive diffusion, a transport mechanism which is near efficient over brusque distances. Thus, the cardiovascular system uses majority flow (convection) to reduce the effective distance between the pumping activity of the middle and the various parts of an organism.
In order for this system to be practical and do its job efficiently, two of import conditions must exist satisfied: (ane) there must be adequate claret menstruum through the smallest blood vessels, capillaries, which are in contact with the cells comprising a tissue; and (ii) the chemic limerick of the incoming blood must exist controlled to be that which is desired in the ISF. The blueprint and performance of the cardiovascular arrangement fulfill these atmospheric condition. Two important functions of the cardiovascular arrangement are to movement material (the carrier is blood) and to move heat (tissue metabolism generates oestrus that must be brought from the body's cadre to the cutaneous vascular bed at its surface, where it is radiated away from the torso).
Pattern OF THE CARDIOVASCULAR SYSTEM
The systemic circulation and pulmonary circulation are connected in series through the four chambers of the center, so that all the blood that is pumped from the left ventricle into the systemic organs somewhen makes its manner back to the right ventricle from where it is pumped into the lungs. The systemic organs (tissues) are continued in parallel, and the following statements are consequences of this parallel compages: (1) the stroke book ejected from the left ventricle is divided among the various organs, and a given book of blood passes through but one organ earlier entering the venous outflow of the organ; (two) the arterial blood entering each organ has the same limerick; (3) the blood pressure at the entrance to each organ is the same; and (4) the blood menses to each organ can be controlled independently (local regulation of blood catamenia).
The various organs and tissues can be classified as one of two broad types: (1) claret "reconditioners" and (ii) "essential" tissues. The main purpose of the blood "reconditioners" is to maintain the composition of the ISF relatively constant under all atmospheric condition. In general, flows to these tissues exceed their metabolic needs. Examples of this type of tissue are the lung, which ensures proper substitution of oxygen and carbon dioxide; the kidney, which maintains electrolyte composition and fluid balance; the gut, which oversees food absorption; and the skin, which is involved in temperature regulation. The "essential" tissues are those whose function is critical at all times. The blood flows to these tissues typically match their metabolic needs. Examples of this blazon of tissue are the centre, which requires a continuous supply of energy to maintain its pumping activity, and the encephalon, which requires a continuous supply of nutrients and a demand for the washout of metabolic products in order to maintain consciousness and carry out its critical functions. One tin can as well add skeletal musculus during practise to this list, since its energy requirements and needs for washout of metabolic products tin exist substantial.
HEMODYNAMICS
A requirement for the circulatory organization to carry out its part of bringing blood close to cells and so that the commutation of nutrients (e.g., oxygen) and wastes can have place past diffusion is that the blood be able to period through the complicated networks of blood vessels in the various organs. In order to make a viscous fluid such equally blood menstruation, whether through a single vessel, an organ or the entire systemic circulation, a pressure divergence must be applied between the arrival and outflow of the network. The relationship between volumetric flow, Q, and the applied pressure level difference, ΔP, is the fluid mechanical equivalent of Ohm's police for electrical circuits and is expressed every bit Q = ΔP/R, where R is the resistance to the catamenia of claret. Although the myriad of serial and parallel connections of blood vessels in a tissue is quite complicated, each element—a single vessel segment—is uncomplicated to deal with.
Flow of Blood Through Single Vessels
Poiseuille'south law for a viscid fluid quantifies the human relationship amidst the volumetric flow of blood through a claret vessel, modeled as a circular cylindrical tube, the geometric properties of the tube and the catamenia properties of the blood. Poiseuille's police (1846) is normally expressed as:
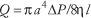
where Q is volumetric flow, the cistron π/8 arises from the circular cross-department, a is the radius of tube, fifty is the length of tube, η is the viscosity of the blood, and ΔP is the force per unit area deviation between the ends of the tube, also chosen the driving pressure level or perfusion force per unit area. It is noteworthy that the fourth power dependence of flow on radius means that blood flow is quite sensitive to changes in radius, which can vary in the circulatory organisation every bit vasomotor tone in vessels decision-making period (i.e., mainly arterioles) changes. It should also be noted that vessel length is generally constant for a given vessel and that viscosity is a belongings of claret related to the ease with which information technology tin can be made to flow. From the human relationship among Q, ΔP and R, i finds that R depends on the geometry of the vessel and the viscosity of the blood as
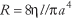
The average velocity of blood through a vessel tin can also be expressed in terms of the to a higher place factors. Conservation of menses leads to the conclusion that volumetric period is equal to the product of average velocity, v, and the cross-sectional area of the vessel, πa 2:
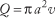
Thus, the average velocity tin can be expressed equally

For a Newtonian fluid flowing through a vessel or tube of circular cross-section, the radial dependence of velocity is described as "parabolic" due to the quadratic dependence of velocity on radial position, r:
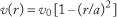
where a is the internal radius of the tube, and v 0 is the maximum velocity that occurs on the centrality (r = 0); the minimum velocity is zero at the wall (r = a; called the "no slip" condition).
Construction AND Function OF THE MICROCIRCULATION
The microcirculation deserves special attending since it is beyond the walls of these vessels that the exchange of oxygen, amidst other substances, takes place [82]. Furthermore, the arterioles, as well known as the "resistance" vessels, are the main site for command of blood catamenia. Thus, the claret vessels of the microcirculation play important roles in both the convective (arterioles) and diffusive (capillaries) transport of oxygen. These blood vessels are classified as arterioles, capillaries and venules and vary in diameter from about 100–200 μm for the largest arterioles and venules downwards to about v μm for capillaries. In terms of their structure, all these vessels possess an inner layer of endothelial cells. In addition, the arterioles have a circumferential layer of vascular smooth musculus with which they can control blood flow and its distribution inside organs. Venules typically have thinner layers of polish muscle.
The chief office of the circulatory organisation is to exchange substances betwixt blood and tissue, and these substitution processes accept identify in the microcirculation. The classes of vessels playing a role at that place are the arterioles (resistance vessels which regulate flow), capillaries (the master exchange vessels) and venules (commutation and collecting vessels). The amount of flow through the capillaries appears to exist regulated to maintain adequate tissue oxygenation. The regulation of blood flow appears to be accomplished by the coordination of several different mechanisms which bear on the flow of blood through precapillary vessels.
TRANSCAPILLARY Commutation OF SOLUTES
The transport mechanism of passive diffusion is a rapid and efficient mode of molecular exchange over the small distances (tens of micrometers) between the blood supply (capillaries) and tissue cells. Fick's first law of diffusion (1855) describes the net rate of transfer of a substance from a location of high concentration to i of lower concentration:

where ΔNorthward/Δt is the amount of the substance exchanged per unit time, D is the diffusion coefficient for the substance through the capillary wall, A is the surface area available for diffusion (proportional to the number of claret-perfused capillaries), Δc is the concentration deviation across the capillary wall or Δc = c(blood) − c(ISF), Δten is the thickness of the capillary wall (∼i μm) and P is the permeability of the capillary wall divers as D/Δx.
In regard to the permeability characteristics of the capillary wall, the wall is composed of a single layer of endothelial cells about 50 μm thick. For lipid-soluble substances (eastward.yard., oxygen), the entire wall surface is available for diffusion. For water-soluble substances (e.g., glucose), there are pocket-size aqueous pathways equivalent to cylindrical pores 80 to 90 Å in diameter through which they may laissez passer. Full pore area is about i/yard (i.eastward., 0.1%) of the expanse of a typical capillary. The permeability of the wall to a item substance depends upon the relative size of the substance and the pore ("restricted" diffusion).
During times of increased action in a tissue, there is a need for delivery of more nutrients to the active tissue, equally well equally a need to eliminate accumulated metabolic wastes that result from the increased metabolism of the tissue. The amount of a substance which is exchanged between claret and tissue can be increased by having more of the anatomically present capillaries perfused with claret. This increases the surface area available for commutation and reduces the distance that exchanged molecules must diffuse, both of which increase the efficiency of improvidence. At that place is some controversy regarding whether it is the number of blood-perfused capillaries that is important or, in the case of oxygen exchange, whether it is the area of the capillary wall in contact with moving cherry claret cells. Nether resting, baseline atmospheric condition, the equivalent of only a fraction (nearly 1/3 to 1/2) of the capillaries in a given tissue are beingness perfused at any given moment. During times of increased demand for nutrients and particularly oxygen (e.g., heart and muscle tissue during do), more than capillary pathways tin can be opened to flowing red blood cells. Whether a given capillary is open or closed depends on the contractile state of a region of smooth muscle (probably a concluding arteriole) located near the entrance to a capillary [52].
REGULATION OF Claret Menses
Since the convective supply of oxygen depends directly on blood period, the regulation of tissue oxygenation depends critically on the regulation of blood flow. The cardiovascular system controls blood menstruation to individual organs (1) by maintaining the input pressure to each organ within narrow limits by the mechanisms designed to regulate arterial pressure and (2) by allowing each organ to arrange its vascular resistance (R) to blood flow to an advisable value. The cardiac output (CO) is distributed among the diverse organs according to their respective resistances then that flow (Q) in an organ is given by:
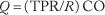
where TPR is total peripheral resistance of the systemic circulation. There are three major mechanisms that command the function of the cardiovascular system: local, neural and humoral. They can work independently of each other, but in that location are also interactions among them. The local mechanisms are intrinsic to a tissue and will be described in more particular below. The neural mechanisms involve the central nervous system and rely primarily on the release of norepinephrine from the sympathetic nerve endings of the autonomic nervous arrangement. Finally, the humoral mechanisms rely on circulating vasoactive hormones, such every bit angiotensin Two and epinephrine. Information technology is important to recognize that the vasoregulation occurs in the resistance vessels. In the context of the regulation of tissue oxygenation, information technology is most appropriate to focus on the mechanisms that command blood flow at a local level.
Local Regulation of Blood Flow
The local mechanisms for regulating claret menstruum are intrinsic to the various tissues and can operate independently of neurohumoral influences [xiii,91]. Local regulatory processes allow each tissue in the torso some measure of autonomy to satisfy its electric current and particular requirements in regard to blood period. Because the various organs and tissues of the body are continued in parallel, the cardiac output can be redistributed among the tissues should their relative need change past altering the resistance (R) to blood flow in the affected tissues.
The site of local regulation of blood flow is the microcirculation, which is composed of a network of blood vessels—arterioles, capillaries and venules—whose functions are regulation oftissueperfusion and exchange of substances between claret and tissue. Although the topology of vascular networks is typically quite complex, equally a first approximation, i can recall of most networks as a collection of microcirculatory "units" connected in parallel, where each unit is composed of a feeding arteriole, several capillaries arising from the arteriole and a venule which collects the blood after molecular exchange has taken place between information technology and the interstitial fluid. Because of the parallel structure of the network, which is a collection of these microcirculatory units, it is possible to redistribute blood flow from one region to another within a tissue to suit any alterations in local metabolic needs.
Examples of local blood menstruation command processes are autoregulation, reactive hyperemia and active (or functional) hyperemia. The term "autoregulation" in this context refers to the tendency for organ blood period to remain abiding in the face of local changes in arterial or perfusion pressure. Autoregulation is observed in near every vascular bed. It is near pronounced in the brain and kidney and is prominent in the heart, skeletal musculus, intestine and liver. Call back that menstruation (Q) equals perfusion pressure (ΔP = difference between inflow arterial pressure and outflow venous pressure, P a − P v) divided past vascular resistance (R) and so that, every bit ΔP rises through the autoregulatory range (P a ≈ 80–160 mm Hg in brain and kidney), R must increase to maintain constant flow. Reactive hyperemia refers to the elevated blood catamenia observed in an organ when menses is restored following a period of circulatory abort (i.east., apoplexy of the blood supply). Hyperemia is literally an backlog of claret in a region. The magnitude of the hyperemia is related both to the duration of the occlusion period and to the pre-occlusion blood flow. Active (or functional) hyperemia refers to the increase in blood flow which accompanies an increase in the metabolic activity of an organ or tissue. It has been described in skeletal and cardiac musculus, brain, intestine, tummy, salivary glands, kidney and adipose tissue. The name of the hyperemia depends upon the specific function of the tissue (e.g., contraction hyperemia for musculus or secretory hyperemia for various glands). Each one of these examples of local regulatory processes can be linked to the regulation of tissue oxygenation.
Mechanisms of Local Regulation
Two major mechanisms have been proposed to account for the local regulatory phenomena described above: the myogenic mechanism and the metabolic machinery. Although these mechanisms appear to act independently, the expression of each mechanism varies amongst tissues and some combination of each one is probably operative, depending on the detail intervention, i.e., altered perfusion force per unit area, flow or tissue action.
Myogenic Mechanism
The myogenic mechanism, in essence, states that vascular shine musculus actively contracts in response to stretch, in an endeavor to maintain circumferential wall tension, T, relatively abiding in the resistance vessels. The human relationship among wall tension (T), intravascular force per unit area (P), internal radius (a) and vessel wall thickness (due west) is given by the law of Laplace (1805) for a cylindrical elastic tube: T = Pa/w. Thus, elastic blood vessels exposed to an increased intravascular pressure will become passively distended. The smooth muscle in the vessel wall responds by agile contraction (leading to vasoconstriction) which tends to return wall tension near its baseline value and vascular caliber below its original value. The myogenic mechanism is sometimes referred to every bit pressure level-related command of claret flow.
Metabolic Machinery
The metabolic mechanism states that there is a close link between blood flow and tissue metabolism. It has usually been specialized to suggest a link between oxygen supply and demand co-ordinate to Figure i.
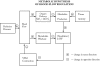
Effigy one
Schematic diagram of metabolic machinery for blood menses regulation. Arrows between boxes are each associated with a "+" or "−" sign which indicates that the direction of change in the "downstream or effect" (more...)
Tissue cells continuously apply ATP as an free energy source to maintain cellular part. The ii most common ways in which ATP can be produced are by oxidative phosphorylation and glycolysis. Since oxidative phosphorylation is the preferred pathway for most cells to generate ATP, cells have a continuous need for oxygen. In the presence of an adequate supply of oxygen (normoxia), the adenosine diphosphate (ADP) produced from the hydrolysis of ATP is rephosphorylated every bit role of the process of oxidative phosphorylation, and the contribution of glycolysis to ATP production is negligible. When the supply of oxygen decreases beneath normal (hypoxia), not all of the ADP is rephosphorylated, and some is degraded further to adenosine monophosphate (AMP) and then to adenosine. Adenosine is a powerful vascular smooth muscle relaxant (i.eastward., produces vasodilation), and the corporeality of information technology produced is tightly linked to the degree of hypoxia. During hypoxia, glycolysis is stimulated, and some of the lost mitochondrial ATP production is made up through this metabolic pathway. The finish product of glycolysis, lactic acrid, dissociates into hydrogen ion and lactate, both of which as well have vasodilator properties. A general principle then is that cells continuously produce metabolic wastes (eastward.g., adenosine, hydrogen ion, lactate), many of which are vasoactive (usually vasodilator). Metabolite production occurs at a low level, even nether normoxic atmospheric condition. There appears to be a close linkage between metabolite production and tissue oxygenation, so that metabolite production increases equally tissue oxygenation decreases, and vice versa. The master reason responsible for a decrease in metabolite production with increases above baseline in tissue oxygenation is that a small fraction of most tissues are slightly hypoxic at any moment, merely temporal variations in the regional distribution of tissue perfusion practice not allow situations of chronic hypoxia to develop. Under normal conditions, there is a balance between oxygen supply and need, merely imbalances give rise to local adjustments in blood catamenia that bring supply back in register with demand.
The following oxygen-linked metabolites have been implicated every bit potential chemical mediators in the metabolic mechanism of claret flow regulation: adenosine (from ATP hydrolysis: ATP → ADP → AMP → adenosine) and hydrogen and lactate ions (from lactic acid generated by glycolysis). The levels of these metabolites are increased when there is a reduction in oxygen supply relative to need, leading to tissue hypoxia. The production of more than carbon dioxide as a result of increased tissue activeness (leading to increased oxidative metabolism) leads to vasodilation through increased H+ since carbon dioxide reacts with water to class carbonic acid which rapidly dissociates into H+ and bicarbonate ion. Increased release of potassium ion and increased interstitial fluid osmolarity (i.e., production of more than osmotically agile particles) transiently cause vasodilation under physiological conditions associated with increased tissue activity.
Two of the components in the block diagram above deserve further description. The cake labeled "Oxygen Delivery, Q O2 = Q [O2]a" refers to the convective flow of oxygen in the arterial blood. Thus, oxygen is delivered by bulk flow of blood (flow = Q) to the commutation vessels (i.e., capillaries) by virtue of its presence in the blood at concentration [Oii]a. Hence, increasing claret period will increase the delivery of oxygen via the claret to the tissues. The cake labeled "Metabolite Washout" plays a primal part in determining the concentration of vasodilator metabolites in the interstitium, [Vasodilator]ISF. The concept of metabolite washout can be appreciated by considering the motility of the vasodilators produced in tissue cells. They diffuse away from their sites of product, through the interstitial fluid and across the walls of the nearby capillaries (these molecules are generally small enough to pass through the aqueous channels in the capillaries; and most cells have at least one capillary near them). Once the metabolite enters the capillary, it is "washed away" by the blood flowing through the capillary, hence the term "metabolite washout." It is the summation of metabolite production and metabolite washout that determines the concentration of vasodilators in the ISF in contact with nearby arterioles that control blood flow. Increases in metabolite concentration thus cause vascular smooth musculus relaxation, lowering the resistance to blood flow. Consider exercising skeletal musculus as an example. With the onset of practice, metabolite production and oxygen requirements both increase. The vasodilator metabolites diffuse away from their sites of product and attain the resistance vasculature through the interstitial fluid. Vasodilation ensues, lowering resistance to claret menstruum. The resulting increase in blood period increases the oxygen supply, and finally, a new steady land is achieved in which oxygen supply and need are matched. This scenario operates for other tissues in which metabolic activeness changes.
Other Oxygen-Linked Mechanisms of Menses Regulation
Several other issues related to the regulation of blood menstruum, and hence convective oxygen delivery, volition be considered hither since they take a direct affect on the regulation of tissue oxygenation. The question arises equally to whether tissue P O2 closely regulated and whether oxygen plays a straight office in oxygen-linked flow regulation by interim direct on the vascular smooth muscle of resistance vessels. Duling and Berne [xviii] found that tissue P Oii was regulated within a narrow range, even when the P Oii of a superfusion solution was varied over a relatively broad range of tens of mm Hg. Thus, raising the P Oii of the superfusion solution led to arteriolar constriction, but a relatively constant tissue P O2 , suggesting that some components of the tissue and/or the arteriolar wall were sensitive to oxygen and communicated with the arterioles to limit blood flow and oxygen delivery to a desired level. Furthermore, subsequent experiments using a bicarbonate-buffered superfusion solution showed that in the presence of carbon dioxide, tissue P O2 was however regulated but at a higher P Otwo than in the absence of carbon dioxide [fifteen]. Duling [15] suggested that there were two possibilities that might explain this finding: (1) the oxygen supply might exist regulated by a directly result on vascular smooth muscle or (ii) oxygen acts only through cellular metabolism and that the rate of oxygen delivery to cells, rather than the absolute P Oii , would be regulated. Further in vitro [75] and in vivo [16] experiments raised serious doubts that vascular smooth muscle had the requisite sensitivity to oxygen, and then that the blood and nearby tissue would demand to become severely hypoxic (P Oii of only a few mm Hg) before arteriolar shine muscle would answer with relaxation. Cytochrome c oxidase, which has a high affinity for oxygen (depression K M or P 50 of ∼1 mm Hg) and is thus responsive to oxygen levels over a narrow range, was considered to be the oxygen "sensor" in the above studies. Duling [17] afterwards considered that other oxidases and oxygenases, with college 1000 1000'due south or P l'southward and which serve other oxygen-linked processes, might take on the role of oxygen sensor.
The novel concept of a mobile oxygen sensor was put forth by Ellsworth and colleagues [24]. The idea is that, when the hemoglobin in RBCs becomes partially deoxygenated, there is a conformational change in the hemoglobin molecule that is transmitted to the RBC membrane and leads to the release of ATP from the RBC. The ATP binds to purinergic receptors on the endothelial cells and results in the relaxation of arteriolar smooth musculus cells, increased blood flow and increased oxygen delivery. A substantial amount of supporting data take accumulated in favor of this ways to regulate blood flow, and the idea of a mobile, oxygen-linked sensor that is intimately associated with the carriage of oxygen itself is peculiarly attractive.
Is tissue P O2 really regulated in the sense of being a controlled variable in a feedback loop which involves a chemical mediator? There are multiple redundant systems which play roles in modulating blood menstruum [13], and an oxygen-linked system is simply i of many. However, the maintenance of tissue oxygenation is such an important feature for survival of the organism that it seems necessary that some mechanisms must exist to ensure an acceptable oxygen supply to all cells of the organism. Possibly at that place is some overall coordination of events which regulates blood menstruation to ensure removal of metabolic wastes and delivery of oxygen and nutrients, and leads to an oxygen level consistent with maintaining a balance between energy demand and production.
Propagated Vasomotor Responses
It has been found that the local vasomotor responses can spread from their indicate of origin to upstream and downstream sites by electric conduction through gap junctions betwixt endothelial and vascular smooth muscle cells [two,91]. Since the metabolic responses most closely associated with the regulation of tissue oxygenation will be expressed and sensed initially in the terminal branches of the microvascular network (i.e., capillaries and terminal arterioles), their spread to upstream sites volition typically pb to increased blood flow, and hence oxygen supply, through increased vasodilation of arterioles. Thus, the local signals confined to peradventure tens of micrometers can exert their influence over a much wider spatial domain of hundreds to thousands of micrometers, thereby recruiting many vessels in the network to participate in the hyperemia. The sensitivity of the vascular wall to diverse locally produced vasoactive substances (archetype metabolic mechanism), shear stress (flow-induced release of NO from the endothelium) and stretch (myogenic mechanism) appears to vary along the vascular network and from organ to organ. The propagated vasomotor responses thus act to coordinate and integrate the regulation of tissue oxygenation.
What Controls The Direction Of A Molecule, Such As Oxygen, Involved In Passive Transport?,
Source: https://www.ncbi.nlm.nih.gov/books/NBK54112/
Posted by: hillbroged.blogspot.com


0 Response to "What Controls The Direction Of A Molecule, Such As Oxygen, Involved In Passive Transport?"
Post a Comment